
Turning the Table
by Judy Purdy

Necessity, sure.
But frustration can be a mother of invention too, having led a UGA geologist to devise an alternative table of the elements.
“The conventional periodic table wasn’t doing what we needed it to do,” said Bruce Railsback, the tool’s inventor. “I found myself teaching next to the conventional periodic table with my arms crossed, pointing at things that should have been together. And that’s what got me started.”
After investing countless hours combing the literature for information and discussing ideas with colleagues, Railsback devised a table for Earth scientists. It provides rich context on patterns, relationships and trends among naturally occurring elements in the Earth’s crust, mantle, oceans, atmosphere and even living organisms. While the conventional table arranges elements by atomic mass, Railsback’s version organizes them by ionic charge — or number of shared electrons — meaning that several elements appear more than once.
For three years, the inventor struggled to find a publisher. When the journal Geology finally issued “An Earth Scientist’s Periodic Table of the Elements and Their Ions” in September 2003, no one anticipated its instant popularity. Within two months Geology’s parent, the Geological Society of America, had sold all 1,400 extra copies, fueled by stories in Nature and other journals.
In recognition of Railsback’s innovation, Discover magazine ranked the table among its Top 100 Science Stories of 2003.
Organizing elements by charge means that natural groupings emerge, based particularly on how ions bond with oxygen. That’s an important property to geologists because oxygen, abundant in the Earth’s atmosphere for the past two billion years, was once scarce. Oxygen thus becomes increasingly rarified the deeper one probes beneath the planet’s surface. Ions on the table’s left side are found in geologically younger materials on or close to the surface; those on the right date to the planet’s earlier days and are more typical of its mantle.
The table also predicts such things as the occurrence of elements in soils and sediments, their abundance in atmospheric gases and their ability to dissolve in water. Aluminum and titanium, for example, “are miles apart and seemingly have nothing to do with each other” in the conventional table, Railsback said. In the geologist’s table, however, they appear together as part of a mineral-forming group.
Other relationships also emerge, such as elements essential for vertebrate nutrition or those that can’t be transported across cell membranes.
And the new tool makes clear why elements such as sulfur can dissolve in water and form tight bonds in fire-formed igneous rock. A vivid yellow powder in its elemental form, sulfur can share up to six electrons. Sulfur ions on the table’s left side form weak bonds — as sulfates they dissolve in seawater (symbolized by a blue beaker), and make soluble minerals. On the right side, the ions are sulfides, which can form minerals (symbolized as yellow and orange diamonds) such as iron sulfide, also known as pyrite or fool’s gold.
“Geologically, we need to talk about something like sulfur in these different guises because that’s the way it works in the real world,” Railsback said.
More than a teaching tool, his innovation should be useful for research. “I hope that as people look at the table,” he said, “they’ll see interesting questions that ought to come up.”
For more information, email Bruce Railsback at rlsbk@gly.uga.edu or access http://www.gly.uga.edu/ railsback/PT.html.
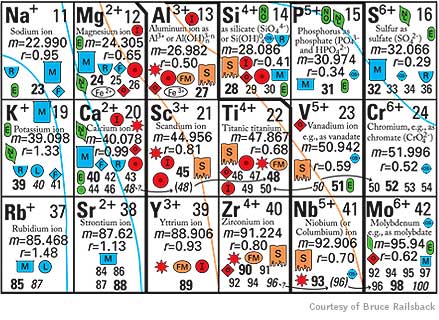
This section of "An Earth Scientist's Periodic Table of the Elements and their Ions" shows how elements are organized by ionic charge. The table provides an array of information, from an element's water solubility to its occurance in soils.
For comments or for information please e-mail the editor: jbp@ovpr.uga.edu
To contact the webmaster please email: ovprweb@uga.edu
![]()