 |
Research Magazine > ARCHIVE > Summer
97 > Article
Suicide: A Strategy for Life
by Judy Boylard Purdy
By sacrificing a few healthy cells, animals ensure normal growth and development
and can thwart disease.
Every moment of every day, some of the cells in your body are killing themselves
so that you can live.
Cell suicide is a routine, essential process. It's how tadpoles lose their
tails and how babies develop fingers and toes. It's how our bodies stymie tumors
and fight back viral invasions. It's the garbage collection system that eliminates
diseased or unnecessary cells, such as milk-secreting mammary cells after a
baby is weaned.
This basic survival strategy is hard-wired into our genes: Sacrifice a few
cells for the health and development of the organism.
But let something go awry - a few too many, or maybe too few, cells destroy
themselves - and the system fails. The result: diseases like cancer, rheumatoid
arthritis, Alzheimer's and AIDS.
That's why Lois Miller, a UGA Research Professor of Genetics and Entomology
and a fellow of the National Academy of Sciences, has devoted the past seven
years to the study of cell suicide, which has become one of the hottest topics
in cell biology.
Technically, the process is called apoptosis (a puh TOE sis). But it is not
the same thing as necrosis, which is what happens when cells die from injury.
Apoptosis is neat, controlled, orderly and self-contained; necrosis is violent
and explosive, usually causing inflammation and swelling.
"What's so remarkable about apoptosis is that it was ignored for so long
and it is so essential to everything," Miller said. "People think only
of life, but in fact death is important in the proper balance of life."
Even though the term apoptosis, which is derived from the Greek for "dropping
off," as in leaves dropping off trees, was first introduced in the scientific
literature in 1972, the concept has only gained acceptance among scientists
in the past couple of years. It's so new that you won't even find it in the
latest biology textbooks.
"It wasn't appreciated that apoptosis was a central battle," said Miller,
who receives grants totaling about a half million dollars annually from the National
Institutes of Health to support her internationally recognized research programs
on apoptosis and biopesticides. "It was known that a major battle is fought
when a virus tries to establish an infection."
Suicide as a defense mechanism
When a virus invades the cells of a host organism, its purpose is to take over
the metabolic processes of the host cells and get them to make more virus instead.
"Viruses are amazing organisms," Miller said. "They express genes
in just the right sequence and take over cell machinery to start making more
of their own kind."
Cell suicide is one way host cells can foil the virus' takeover attempt.
But some viruses have developed the ability to circumvent the host cells' suicide
defense system. Miller was the first to discover this new weapon - an antiapoptotic
gene - in an insect virus. Her discovery was an outgrowth of her research program
to develop a class of insect viruses, called baculoviruses, as biopesticides
for harmful pests like armyworms, budworms and cabbage loopers. These and other
pests feed on corn, tomatoes and other crops, causing much destruction and
damage. Miller's research program, in addition to yielding four biopesticide
patents, has turned up an apoptosis-blocking gene, which she named p35, in
the baculovirus called AcNPV.
While studying the safety of bioengineered pesticides, Miller and graduate
student Rollie Clem noticed something unusual: Some insect cells that had been
infected with a mutated form of the baculovirus AcNPV still were able to commit
suicide. The mutated baculovirus - unlike the normal form, which carries the
p35 gene - is not able to block apoptosis in host insect cells. In 1991, Miller's
team published its preliminary findings - that p35 blocks apoptosis in host
insect cells - in the journal Science.
"It's a race between the virus and the host," she said. "The virus
would evolve to adapt gene expression to beat the host before the host mounts
an apoptotic response. What we showed in this paper was that many of the [insect]
viruses carry at least one gene and maybe more which can block apoptosis."
The ability of a virus to combat programmed cell death in a host organism also
may help a virus extend its range to infect an even broader array of insects.
When Miller and her team, which includes seven postdoctoral fellows and a half
dozen or so graduate and undergraduate students, went looking for similar genes
in the world's databases, they could find none that resembled p35.
"It was unique in the database," she said. "There were no other
known sequences like it."
Subsequent investigations of distantly related baculoviruses turned up two
more apoptosis-blocking genes, both unique and previously unknown. These new
genes, which Miller dubbed inhibitor of apoptosis, or IAP for short, are nothing
like p35.
With two types of apoptosis-blocking genes to work with, Miller turned her
attention to figuring out how cell suicide works at the molecular level. After
all, if an insect virus carries genes that block programmed cell death in insects,
chances are other viruses have evolved similar genes that block cell suicide
in other kinds of animals, including humans.
Sure enough, two years after Miller's team found the first IAP genes in a baculovirus,
three other research groups reported finding IAP genes in cells of mammals
and insects.
Meanwhile, Miller was intent on finding what events in and around a cell determine
whether it lives or dies, because that kind of information would be valuable
in lots of fields: genetics, immunology and molecular biology, to name a few.
"You can't really figure out where inhibitors fit into the apoptotic pathway
unless you understand the pathway itself," she said. "And then you
can figure out where the inhibitors work."
Simple model, complex process
The research team started with the apoptotic pathway of a lowly worm called
a nematode. Like all animals, the nematode starts out as a single cell. As
it develops it loses 131 cells to cell suicide, ending up with about 1,000
cells as an adult.
"In a nematode we're talking about a simple and exquisite system for cell
death study," Miller said. "As systems get more and more complex and
sophisticated, you need many more genes in more and different systems to control
and initiate apoptosis. Nematodes are so simple, but whether you're working with
a nematode or a mammal, I think you'll find the same basic pathway."
Some segments of the nematode's cell suicide pathway were understood. For example,
scientists already knew that nematodes have three central cell death (ced)
genes - ced-3, ced-4 and ced-9 - that control cell suicide decisions. But it
wasn't clear how they interacted until Miller's laboratory began its study.
Understanding how nematode genes control cell suicide at the molecular level
almost certainly will help scientists understand how apoptosis can be regulated
in humans. And this could lead to all sorts of advances in medicine, including
new therapeutic drugs and chemotherapies. New treatments for everything from
viral infections and cancers to autoimmune diseases like lupus, rheumatoid
arthritis and AIDS could be devised.
"The importance of this work lies in understanding how the basic process
works and how it applies to humans because apoptotic genes seem to be conserved
in insects, mammals and nematodes," Miller said. "If you find a gene
for apoptosis in an insect, you'll probably find it in a mammal. It's just that
in a human the [apoptotic process] has been elegantly elaborated."
So far only one ced-3-like gene has been found in nematodes and insects,
while more than 10 genes related to ced-3 have been found in mammals. Nematodes
have
one ced-9-like gene and mammals have many, yet none has been found in insects.
Scientists are now looking for a ced-4 gene Ð which is central in and critical
to the nematode pathway Ð in mammals and insects.
Somasekar Seshagiri, a graduate student in Miller's laboratory, has shown that
the ced-4 protein activates the ced-3 protein, which is stored in an immature
form. The activated ced-3 protein is the executioner of apoptosis. He also
has shown that the function of ced-9 is to block ced-4 by binding to it.
"To regulate apoptosis in a nematode, you absolutely have to have the ced-3
and ced-4 genes," Miller said. It's the balance between ced-3 and ced-4
versus ced-9 that leads to the decision that determines whether the cell will
survive."
Another big question for Miller is where IAP genes fit into the overall scheme
of apoptosis. Scientists now know that humans have at least four IAP genes,
including one called NAIP, which seems to regulate cell death of motor neurons,
the nerves that transmit messages to voluntary muscles and allow you to kick
a ball or pet a cat.
Children who inherit a recessive mutation of NAIP
will develop a fatal genetic disease called spinal muscular atrophy. The
disease causes muscles and the
nerves that activate them to shrivel and die, and the child usually dies
before the age of 10. "It is one of most common recessive genetic disorders
that exists," Miller said.
The UGA research team is working with other apoptotic genes - which are
dubbed "grim," "reaper," "doom" and "hid." So
far, these genes have been found only in the fruit fly. The UGA scientists
have found that IAP genes block the gene doom through some sort of physical
interaction, but "we don't understand how doom and reaper connect to the
pathway that includes ced-3, ced-4 and ced-9," Miller said. "There's
a hole right there, and we don't have the answer yet."
For more information, e-mail Lois Miller at miller@bscr.uga.cc.uga.edu.
Judy Bolyard Purdy is UGA's director of research communications and editor
of Research Reporter. A former naturalist with the National Audubon Society,
she has published a book on medicinal plants and has degrees in biology,
botany and journalism.
Return to Summer 1997 Index
Research
Communications, Office of the VP for Research, UGA
For comments or for information please e-mail the editor: rcomm@uga.edu
To contact the webmaster please email: ovprweb@uga.edu
|
| |

|
|
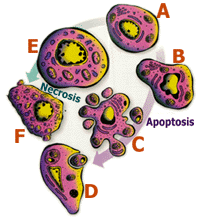 |
Cell suicide, or apoptosis (clockwise arrow), is a deliberate,
orderly and controlled process. A healthy cell (A) shrinks and
its DNA - which usually is dispersed throughout the nucleus - starts
to clump around the nucleus' edge (B). The nucleus and cell quickly
break up, becoming apoptotic bodies (C), which are ingested by
healthy cells nearby (D). Necrosis (counterclockwise arrow) is
a violent and explosive cell death that results from severe injury
and is characterized by swelling and inflammation (E). When a necrotic
cell ruptures (F) it can damage nearby healthy tissue. Illustration
by Terry Hulsey |
|
|


