


Viruses, Vaccines and the "Unstoppable" Ralph Tripp
by Kelli Whitlock Burton
Intro/ The Making of a Biologist
| Seeking Immunity/ Running Interference
| To Avert the Next Pandemic/ Money Talks

![]()
Facility Supports Research on Disease in Animals and People

Seeking Immunity
RSV, a seasonal virus that circulates around the country each year most intensely between October and May, produces an infection that usually begins with a fever, runny nose and cough. Often misdiagnosed as the flu, RSV is the most common cause of bronchitis and pneumonia in young children, and it’s also a major pathogen in the elderly and people who are immune-compromised. A 1999 CDC study found that much of the pneumonia- and influenza-associated deaths in the United States among the elderly actually were caused by RSV.
The infection generally runs its course in a week or two. But in premature infants, the elderly, and people with compromised immune systems, RSV can lead to serious illness. Approximately 90,000 children and adults in the United States are hospitalized each year with RSV, and about 5,000 of them die. The virus does far worse damage in developing countries, where malnourished populations have little defense against infection.
The risk is not one-time-only. If a person infected with a strain of influenza virus survives the illness, the body will develop long-term immunity to that specific viral strain. Not so with RSV. A person can be infected by the same strain of RSV time and time again, and scientists don’t fully understand why. Nor, until recently, had they succeeded in developing any effective RSV treatments, and still no safe and effective RSV vaccine exists.
But in 2001, Tripp and his CDC colleagues identified a protein on the surface of RSV, called G glycoprotein, that attenuates the immune system’s normal response to infection. Their findings, published in the journal Nature Immunology, offered a much-needed peek inside the virus’s chemistry and prompted a change in direction of many labs’ vaccine-development efforts. If the G glycoprotein could somehow be turned off, after all, wouldn’t that give the immune system the boost it needed to defeat RSV?
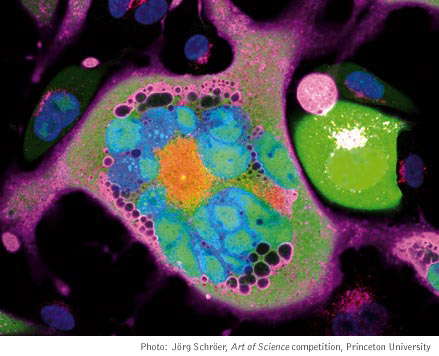
An infected cell (blue) permeated with viral DNA (red and pink).
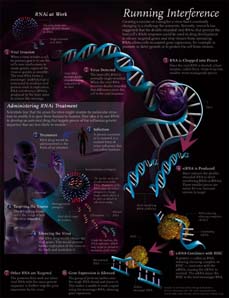
Running Interference
RSV is a paramyxovirus — a virus that relies on its RNA genetic material for replication. HIV, SARS, hepatitis, mumps, influenza and polio are all examples of RNA viruses, and, unlike DNA viruses, RNA viruses are extremely prone to mutations. Creating a vaccine or drug for a virus that’s constantly changing presents a particularly difficult challenge for scientists. But a discovery in 2002 offered a potential new weapon: RNA interference, or RNAi.
First observed in 1990, but not understood until 12 years later, RNAi enables cells to control gene expression, for example to promote or deter growth or to protect the cells from viruses. When a virus invades a cell, its primary goal is to use the cell’s own mechanisms to create genetic copies of the virus as quickly as possible. The virus comes with its own RNA, which it tries to sneak into the cell. (See illustration on p. 18.) But the viral RNA is long and double-stranded while the cell’s RNA normally is single-stranded. That difference alerts the cell to the viral invasion. To protect itself, the cell switches on its RNAi machinery, a complicated network of proteins and enzymes that scientists still are trying to understand. It is thought that once the viral RNA is located, an enzyme called Dicer chops it up into smaller, more manageable pieces. A group of proteins unwinds the two RNA strands, latches onto the single strands and cleaves them. The proteins then look for other viral RNA with the same genetic sequence and destroy them. The effect is the silencing of gene expression by the virus.
During the past five years, scientists have figured out how to replicate RNAi’s defensive process synthetically in models of several organisms, including mammals. Recently, research has suggested that the double-stranded viral RNAs that prompt the host cell’s RNAi response could be used in drug development to silence targeted genes and stop viruses from spreading. At first, many scientists in the field, including Tripp, greeted the new technique with skepticism. But by the time he arrived at UGA, his doubts began to fade because several teams had demonstrated that RNAi worked.
He began organizing a team of scientists at UGA with expertise in RNAi, infectious diseases, vaccine testing and related fields. “We each have strengths in different areas and our personalities complement each other very well,” said Jeff Hogan, a professor of anatomy and radiology who came to UGA in 2004 to collaborate with Tripp. Hogan, who previously worked at the Southern Research Institute, has a background in vaccine development and testing in animal models. Another team member, Mark Tompkins, brings a knowledge of RNAi technology to the group. A professor of infectious diseases, he arrived at UGA in January 2005 after two years of working with RNAi technology at the U.S. Food and Drug Administration.
A virus’s ability to mutate gives it an ability to resist immunity induced by vaccines and drugs designed to stop it. But scientists have learned that most viruses also contain pieces of genetic sequences that are conserved and don’t mutate over time. Seeing them as possible targets for therapeutic drug development, Tripp and his colleagues identified numerous genetic sequences on RSV that seemed to be resistant to mutation.
Late last year, he was contacted by Alnylam Pharmaceuticals, a biotech company in Cambridge, Mass., interested in developing RNAi drugs for respiratory viruses. Impressed with Tripp’s work on RSV, they soon forged a partnership with UGA. Ultimately, his group whittled its list of possible targets to three genetic sequences, which Alnylam then used to develop three RNAi-based drugs, one of which is going into Phase I clinical trials.
RNAi also has potential to “self-vaccinate,” according to Tripp. RNAi treatment of an established virus infection will shut off virus replication but will still provide the host the opportunity to develop an immune response against remaining viral proteins. As a result, RNAi treatment would not only prevent the disease but also vaccinate the individual. In addition, because a virus is a parasite and contains only minimal genetic information, it must hijack an invaded cell’s reproduction machinery in order to grow and survive. Tom Hodge, a principal investigator in the veterinary college’s infectious diseases department, demonstrated in a recent publication that several host-cell genes are not necessary for cell survival but are needed for replication by some viruses. This discovery prompted Tripp and other researchers in his group to ask if a person infected with a virus could take an RNAi drug designed to silence one or more of those host genes. The virus would be unable to copy itself, and the body would gain a lifelong immunity to that particular viral strain. In effect, such a drug would be an anti-viral that works like a vaccine.
Preliminary studies suggest that this strategy would work, Tripp said, but he also foresees potential obstacles. While it may be possible to get FDA approval for RNAi drugs that silence viral genes, he speculates that the regulatory agency may be wary of approving a drug that shuts down genes in the human host. And, as is the case with RNAi drugs that target viral genes, there’s always the problem of how best to get the drug into the body.
Intro/ The Making of a Biologist
| Seeking Immunity/ Running Interference
| To Avert the Next Pandemic/ Money Talks
For comments or for information please e-mail: rcomm@uga.edu
To contact the webmaster please email: ovprweb@uga.edu
![]()