Web Extra
Nobel Prize Spotlights Basic Research
Twenty years ago, UGA researchers initiated the cloning of the gene for the protein that gives jellyfish their green glow – and many believe started a scientific revolution

Aequorea victoria is probably the most influential bioluminescent marine organism.
Late last year, the 2008 Nobel Prize in Chemistry was awarded to Osamu Shimomura, Martin Chalfie and Roger Tsien for discoveries based upon the use of green fluorescent protein (GFP), the protein that gives jellyfish their green glow.
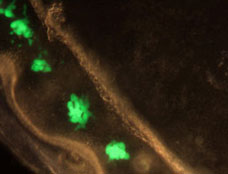
The award received an unusual amount of attention because it’s a classic example of how basic research in areas of science as seemingly esoteric as glow-in-the-dark jellyfish can lead to great breakthroughs. GFP is now a ubiquitous tool used in drug discovery, genetic engineering and most types of bioscience research. GFP makes it possible for researchers to watch biological processes, such as the development of nerve cells in the brain or how cancer cells spread. Simply by shining ultraviolet light on cells that have been “tagged” with the protein, researchers can now see what was previously invisible.
I was grateful that GFP received such international acclaim because over 20 years ago basic research in my lab at the University of Georgia initiated the cloning of the GFP gene – a key event in the history of GFP. Of course, not everyone associated with a discovery can receive a Nobel – that distinction is given to those few who make the greatest contribution. But it’s important to recognize that many scientists – researchers, graduate students, post-docs, their advisors and supporters – working in many different places, over many years, contributed to the understanding of GFP, the protein that many believe started a scientific revolution.
The 2008 Nobel award recognized Shimomura for his 1962 discovery of GFP derived from the luminescent jellyfish Aequorea, and Chalfie and Tsien for their elegant gene expression experiments with the GFP gene beginning in the late 1990s. My own enthusiasm for bioluminescence began in the 1950s when I was a graduate student in biochemistry at the Oak Ridge National Laboratory in Tennessee, attempting to understand the chemistry that caused a bacterium to produce its beautiful blue glow. In 1953 and 1954, Bernard Strehler and I discovered two of the chemicals involved, and we were able to duplicate the blue luminescence in a test tube. I knew then that bioluminescence was to be my life’s work.
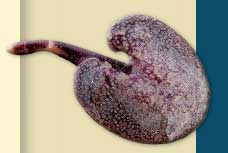
At the University of Georgia in 1958, I worked on understanding the bioluminescence system of the marine invertebrate Renilla reniformis, also known as the sea pansy, a soft coral found in the shallow waters of coastal Georgia. When excited either by tactile or electrical stimulation, Renilla produces a beautiful green luminescence that travels rapidly across the surface of the animal and up the polyps.
In the late 1970s, members of my laboratory isolated and characterized proteins involved in Renilla luminescence. John Matthews, a graduate student, isolated the enzyme Renilla luciferase in 1977. John Wampler, a postdoctoral fellow in my lab, did early work on the purification and properties of a second protein, Renilla GFP. Final purification of Renilla GFP, published in 1979, was accomplished by Bill Ward, who was also a postdoctoral fellow in my lab.
But Renilla is only one of many marine organisms with illuminating properties. In 1962, Shimomura at Princeton identified a protein from the jellyfish Aequorea, a small translucent, umbrella-shaped invertebrate that produces blue bioluminescent light around its edges when it interacts with calcium. He called this protein aequorin. Our own investigations showed that the chemistry of light emission from aequorin was the same as that from Renilla. Shimomura also identified a green fluorescent protein from Aequorea, which he termed Aequorea green fluorescent protein (GFP). GFP from Aequorea is similar but not identical to Renilla GFP. As in the Renilla system, aequorin produces blue light but in the jellyfish, green bioluminescence is observed.
However, biotechnology of the 1980s was not what it is today. Understanding what made the jellyfish glow green required much greater quantities of proteins from the live animals – grams, not milligrams. Two people from my lab went to Friday Harbor, off the Washington coast, to collect the thousands of bioluminescent jellyfish Aequorea needed for large-scale reproduction. One was postdoc Bill Ward. Another was Douglas Prasher, who had just finished his genetics postdoc at UGA.
Doug isolated the first gene coding for the bioluminescent protein aequorin, with lab technician Rick McCann. I will always remember that day: A loud shout from Doug and Rick came across the room, “There are tons of light here. It’s off scale!” That indeed was an exciting day in the lab. We knew that the aequorin gene was being expressed.
We published the first example of cloning of a gene coding for a bioluminescent protein in 1985. Around the same time, graduate student Walt Lorenz was able to clone the gene coding for Renilla luciferase. Just as Doug had done, Walt went on to express recombinant luciferase, making it possible for us to easily purify large amounts of protein. That was another exciting day. Walt received his doctorate for publishing that work in 1991.
After he had cloned the aequorin gene, I suggested to Doug that he try to isolate the Aequorea GFP gene, and after obtaining a clone representing 70 percent of the GFP gene, he moved to the Woods Hole Oceanographic Institute in Massachusetts. We continued to collaborate, and Doug quickly succeeded in cloning the full length gene for GFP. We published this work in the journal Gene in 1992.
Shortly thereafter, Doug left Woods Hole, but before he did, he gave the GFP gene to Roger Tsien at the University of California, San Diego, and Martin Chalfie at Columbia University, who had requested a copy after hearing about our work. Their subsequent classic experiments on gene expression in living cells using the GFP gene as a marker led to them sharing, with Dr. Shimomura, the 2008 Nobel Prize in Chemistry.
Aequeora and Renilla are just two of many species of sea creatures that exhibit the phenomenon of bioluminescence. Recently, other examples of the cloning of genes coding for bioluminescent proteins have emerged, and no doubt there will be future applications from these discoveries. Where they will lead, we don’t yet know. That’s the beauty of basic research.
Milton Cormier is Distinguished Research Professor Emeritus of Biochemistry.
 |