Troubled Waters:
Our Changing Seas
UGA marine scientists study the effects of climate change on Earth’s oceans.
 Whether we dwell along the coast or far inland, the oceans profoundly affect our lives. They help feed us, generate at least half of the oxygen we breathe, and play a key role in regulating Earth’s climate. Governing these and other critical services is a myriad of natural systems and processes such as powerful ocean currents and the intricate carbon, sulfur, and nitrogen cycles. Large disturbances in these natural phenomena could have great impacts on marine life and on human life as well.
Whether we dwell along the coast or far inland, the oceans profoundly affect our lives. They help feed us, generate at least half of the oxygen we breathe, and play a key role in regulating Earth’s climate. Governing these and other critical services is a myriad of natural systems and processes such as powerful ocean currents and the intricate carbon, sulfur, and nitrogen cycles. Large disturbances in these natural phenomena could have great impacts on marine life and on human life as well.
Unfortunately, such disruptions are now occurring because of global climate change, which may be increasing the temperature and acidity of seawater and altering atmospheric and oceanic circulation. It may also be changing vast populations of bacteria and other microbes that play essential roles in ocean processes.
Given the oceans’ vast importance, scientists in UGA’s Department of Marine Sciences are conducting research that could lead to a keener understanding of how climate change affects the oceans—and how the oceans might in turn affect, even mitigate, climate change. Armed with such information, policymakers may be able to develop effective strategies to predict, prevent, or diminish climate change and its impacts.
“The knowledge that marine scientists—and society—gain from our studies will improve the chances that we make the right choices in our efforts to cope with a future climate that may be very different from what we have known,” said department head Tim Hollibaugh.
Much of UGA’s ocean-related climate change research is being conducted under the Georgia Coastal Ecosystem Long-Term Research project, which Hollibaugh founded and now involves many of the marine sciences faculty. Funded by the National Science Foundation, the GCE-LTER project aims to understand how climate change, sea-level rise, population growth, and other factors could affect Georgia’s coastal cities, estuaries, and salt marshes.
A carbon “sink,” with limits
UGA’s marine scientists are concerned with the global picture as well. Their current research might take them as far as Earth’s frozen polar regions or to the mighty Amazon River, where the investigations could help illuminate the situation here in Georgia and across the planet. For example, Patricia Yager examines how warmer oceans will interact with marine ecosystems in the coastal Antarctic as well as with tropical rainforests and rivers. Ming-yi Sun investigates how declining amounts of sea ice in the Arctic affects marine food supplies. And veteran marine scientist Mary Ann Moran has gained critical new insight into ocean-borne organisms that process sulfur, a key ingredient in the formation of solar-reflecting (and temperature-moderating) clouds over oceans.
Much of UGA’s marine research, however, is concerned with another element—carbon—and its compounds. According to the vast majority of scientists, climate change is being driven mostly by rising concentrations of heat-trapping greenhouse gases from human activities such as fossil-fuel burning and deforestation. The most important of these gases is, by far, carbon dioxide.
 The oceans actually play a powerful role in regulating carbon dioxide levels, notes UGA marine scientist Wei-jun Cai. In one major mechanism known as the “biological pump,” countless numbers of free-floating microscopic green plants called phytoplankton, living by the millions per square inch in the oceans’ upper layers, use the gas to make food during photosynthesis. When phytoplankton or the marine organisms that consume them die, much of the carbon they ingested sinks with them to the ocean bottom. And although some of the carbon soon makes its way back up to the surface and into the atmosphere as carbon dioxide—part of the carbon cycle—a great deal of the carbon remains “sequestered,” perhaps for thousands of years, in deep ocean sediments, where it is prevented from reentering the atmosphere as a greenhouse gas. Yager’s research in the coastal Antarctic focuses on the efficiency of the pump and its sensitivity to climate—how climate affects the activities of marine bacteria and other microorganisms, and consequently how much carbon the ocean will take up.
The oceans actually play a powerful role in regulating carbon dioxide levels, notes UGA marine scientist Wei-jun Cai. In one major mechanism known as the “biological pump,” countless numbers of free-floating microscopic green plants called phytoplankton, living by the millions per square inch in the oceans’ upper layers, use the gas to make food during photosynthesis. When phytoplankton or the marine organisms that consume them die, much of the carbon they ingested sinks with them to the ocean bottom. And although some of the carbon soon makes its way back up to the surface and into the atmosphere as carbon dioxide—part of the carbon cycle—a great deal of the carbon remains “sequestered,” perhaps for thousands of years, in deep ocean sediments, where it is prevented from reentering the atmosphere as a greenhouse gas. Yager’s research in the coastal Antarctic focuses on the efficiency of the pump and its sensitivity to climate—how climate affects the activities of marine bacteria and other microorganisms, and consequently how much carbon the ocean will take up.
Overall, the oceans absorb more carbon dioxide than they release to the atmosphere, says Cai. In this way, the oceans act as a carbon “sink.” If most of the carbon dioxide being added to the atmosphere by fossil-fuel combustion—some 25,000 million tons each year, by some estimates—were to sink to the ocean bottom, it could potentially reduce global warming. But no one knows for sure how much carbon the oceans can take up. Warmer waters from climate change, for example, could stymie the seas’ carbon-uptake ability.
Some of the most important and fundamental questions facing climate change scientists are these: How much carbon can the oceans sequester? Can the oceans ramp up to absorb the excess carbon dioxide, and if so, how quickly? And, if we can speed up the process, how will it affect the oceans’ health? Cai is seeking the answers, but he warns that while the oceans may help, they will not provide some magic bullet: “Because of their vastness, the oceans may appear to have an unlimited capacity for taking in carbon. There is a limit, though at this point it is unknown.”
Coastal ocean gets more respect
Much of Cai’s research is devoted to the “coastal ocean,” the portion of the sea that includes estuaries and the continental shelf; for example, the coastal ocean off Georgia extends some 60-to-80 miles offshore. Though occupying only 8 percent of the surface area of the global ocean, coastal oceans “pack a big wallop” in processing carbon, said Cai.

One reason is that rivers flowing to the sea deliver massive loads of plant and animal material—so-called organic carbon—from off the land to the coastal waters. There, teeming masses of bacteria and other microorganisms break down the carbon compounds, a task of monumental proportions.
The assumption has been that the coastal oceans, because of their tremendous carbon-processing ability, generate huge volumes of carbon dioxide that end up in the atmosphere. However, based on their studies along Georgia’s shore and elsewhere, Cai, Yager, and other researchers have proposed that coastal oceans at certain latitudes may themselves be major sinks for carbon dioxide. At these locations, coastal oceans may even act as a one-way pump, absorbing atmospheric carbon dioxide and exporting it to the open ocean for sequestration.
Research by Yager and colleagues on the Amazon River “plume,” the area where river water mixes with seawater at or near the river’s mouth, reveals that large tropical river plumes can extend the influence of coastal processes far offshore. “The Amazon River extends our concept of the coastal ecosystems to an ocean area more than twice the size of Texas,” she said. The Amazon and other large rivers—the Mississippi, Congo, Mekong, and Yellow Rivers, for example—may thus serve as critical links between climate change processes not only on land but also in the sea.
To determine more precisely the relationship between the open sea and coastal oceans, Cai has been measuring the carbon-processing activity in estuaries and ocean-margin areas of the southeastern United States. To aid in his endeavor, he has developed sensitive microelectrodes and automated shipboard sensors that provide more accurate measurements of carbon compounds and other substances in seawater. For his pioneering work, Cai received in March 2008 one of UGA’s coveted Creative Research Medals, which recognizes innovative research that focuses on a single theme.
While the research by Cai and others has helped focus attention on the role of the coastal margins in regulating atmospheric carbon dioxide, coastal oceans have not always been accorded such respect. Until recently most of the climate change research related to the marine environment focused on understanding carbon-based processes in the middle of the ocean. But for many marine scientists, coastal oceans may be the next crucible of climate change study.
Feeling the heat
 One of those marine scientists is Samantha (“Mandy”) Joye—another 2008 Creative Research Medal recipient, who was recognized for her “innovative experimental approaches” in assessing the impacts of climate change on biological and geological processes, particularly those involving carbon, in coastal ecosystems.
One of those marine scientists is Samantha (“Mandy”) Joye—another 2008 Creative Research Medal recipient, who was recognized for her “innovative experimental approaches” in assessing the impacts of climate change on biological and geological processes, particularly those involving carbon, in coastal ecosystems.
In one recent study, of a winding tidal creek near the mouth of the Satilla River on Georgia’s coast, Joye and colleague Nathaniel Weston showed for the first time that even small changes in temperature affect the efficiency of super-sensitive microbes that degrade organic carbon in coastal ocean areas. In effect, Joye says, “temperature short-circuits organic matter recycling,” which means that warmer global temperatures could cause a shift in the fate of sediment carbon—and the release of greenhouse gases from sediments.
In research funded by the U.S. Environmental Protection Agency, she has also been trying to determine how the rising sea levels caused by global warming may affect coastal wetlands, particularly salt, brackish, and freshwater tidal marshes. The hypothesis is that inundation from higher sea levels will destroy large swaths of coastal marshlands and disrupt the functions of many of those that survive.
In particular, Joye notes, through a process called “denitrification” the marshes help break down nitrogen compounds. In limited amounts, the compounds act as fertilizers that help maintain estuaries’ productivity. But a problem arises when excess amounts of nitrogen—from leaky septic tanks and sewage treatment plants and from the run-off of farms, golf courses, and suburban lawns doused with fertilizer—end up in the coastal waters.
An oversupply of nutrients in water— what scientists call “eutrophication”—can rob the water of oxygen, cause overgrowth of plants, and choke out other aquatic life. With the human population mushrooming along shorelines and more nitrogen washing into coastal waters, nutrient overload in Georgia’s estuaries and elsewhere is becoming a serious concern.
“If we lose our marshes and the ability to filter out pollutants, eutrophication will become an even bigger problem,” Joye said.
UGA marine scientist Merryl Alber and graduate student Sylvia Schaefer have found that denitrification rates may depend on temperature. Scientists had long thought that about 25 percent of all the nitrogen dumped into a river and its tributaries—both from natural and human-made sources—reached coastal areas, where it can contribute to eutrophication. That was true of estuaries in cooler, northern areas. But when Alber and Schafer studied estuaries in the much warmer Southeast, they found, to their surprise, that only about 9 percent of the nitrogen in a watershed made its way to the coast.
“These observations,” said Alber, “suggest that temperature increases associated with future climate change may well reduce the amount of nitrogen that reaches estuaries, which will have implications for coastal eutrophication.”
Researchers say this is an important consequence because it directly affects the potential for negative impacts associated with eutrophication—harmful algal blooms, depressed oxygen concentrations, nasty odors, and piles of rotting seaweed washed up on beaches. Because of this, climate change acts to counter the impacts of increased nitrogen loading that accompany coastal development and the burgeoning human population.
Microscopic processes, global impacts
What are the fundamental characteristics of the organisms involved in these aquatic processes? And what drives them to do what they do?
That has been the basis of research, much of it done in the coastal waters off Georgia’s Sapelo Island, led by marine microbial ecologist Mary Ann Moran. As a result, she has helped discover genetic components that dictate the functions of several microorganisms that play critical roles in the carbon, sulfur, and nitrogen cycles.
In one recent study, Moran and colleagues showed for the first time that coastal waters are dominated by marine bacteria species capable of processing a wide variety of organic carbon compounds. In effect, the bacteria are “generalists,” not restricted to just one or two narrow functions. The finding runs counter to some scientists’ belief that specific jobs in carbon cycling are largely performed by highly specialized species. The discovery of these “tidewater bacteria handymen,” able to change their functions as carbon availability fluctuates, should help scientists develop more refined models to help predict how climate change will affect carbon cycling in the oceans, says Moran.
In her research, Moran employs techniques in the relatively new field known as “metagenomics,” which bypasses the need to culture marine bacteria to study their genetic make-up. Because marine bacteria won’t grow in laboratory culture dishes, metagenomic methods have become essential for helping scientists analyze the genetic blueprints of the microorganisms.
In another scientific feat a couple of years ago, Moran and colleagues made an important discovery in the cycling of another element—sulfur. The team found an all-important “switch gene” in two groups of common free-floating “bacterioplankton” that process sulfur.
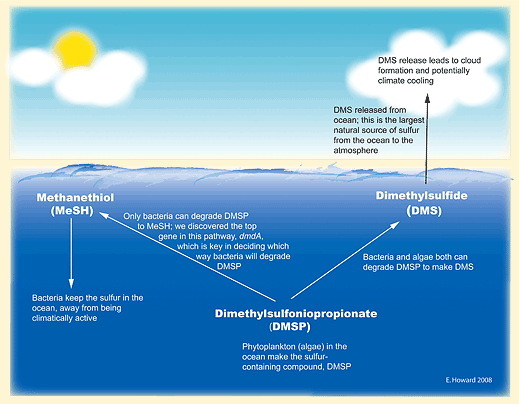
The importance of the bacterial switch gene lies in its power to help control how much sulfur rises into the atmosphere from bacterioplankton. The gene prompts the microbes to convert the sulfur compound dimethylsulfoniopropionate (DMSP), common in seawater, into either dimethyl sulfide (DMS) or into other sulfur compounds. The other compounds remain in the seawater, where they act as nutrients. But DMS rises in huge volumes into the air. There, it can promote the formation of highly reflective clouds, which reduce the amount of sunlight reaching Earth’s surface, thereby helping to offset global warming.
Because this process accounts for almost all of the ocean-generated sulfur in the atmosphere, the UGA team’s accomplishment has opened up valuable new avenues of research. And the main question is: What factor decides whether the bacterioplankton with the switch gene will produce more DMS or the other compounds that stay in the sea? “We need to understand this,” said Moran, “since it would have such important implications for global temperature regulation.”
(Charles Seabrook, a retired science writer for the Atlanta Journal-Constitution, wrote about bioenergy research in the fall 2007 issue of ugaresearch).
 |






