

What Goes Wrong
in Alzheimer's Disease
by Kathleen Cason

![]()
(1) APP extends like a rope from the cell’s interior, through
the cell membrane to the cell’s exterior.
(2) Under normal conditions, the APP protein is cut in two places:
in the cell membrane (g) and outside the cell (a) releasing the active
form of the protein.
(3) Less frequently, the protein is cut at a slightly different
spot (ß) and that makes all the difference. Toxic fragments called
a-beta (red segment from ß to g) are released that can form the
amyloid plaques characteristic of Alzheimer’s disease.
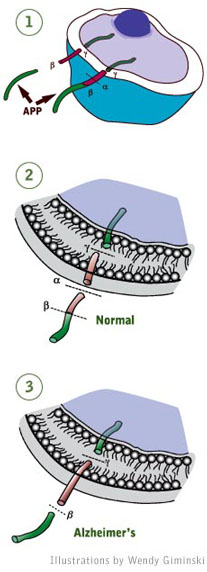
A normal cell protein called APP (or amyloid precursor protein) is a highly
essential protein in the body. But to be active, it must be secreted from
cells and that involves cutting the protein in two spots. Where the cuts
occur determines whether the fragments that are released help or harm a
person.
By the time British author Iris Murdoch felt that she was “sailing into the darkness,” Alzheimer’s disease had long been at work on her brain.
At one time, Murdoch could compose books — in their entirety — in her mind. But in a handful of years, the disease transformed her brilliant intellect to that of “a very nice 3 year old,” according to her husband, John Bayley.
Her dark descent, captured in the recent movie Iris based on Bayley’s books, illustrates the plight of some 4.5 million Americans, a number expected to more than triple by 2050.
What goes so terribly wrong in the brain of an Alzheimer’s patient?
This much we know: In Alzheimer’s disease, lesions — called tangles and plaques — pepper the brain’s memory center like buckshot. The tangles clog nerve cells with gummed up cytoskeletal proteins. Plaques — accumulations of a toxic snippet of a protein — gunk up the spaces between cells. And brain tissue gradually disappears along with the victim’s memories.
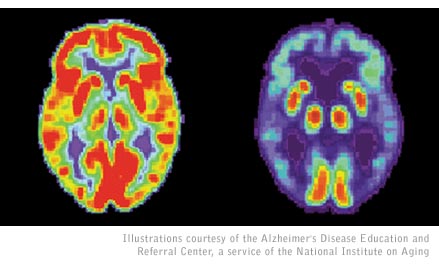
PET
scans show the decline in metabolic activity in an Alzheimer’s brain
(right) compared to a normal brain (left).
“You’re looking at a disease that has been progressing for
30 years,” said Sangram Sisodia, director of the Center for Molecular
Neurobiology at the University of Chicago and a leading Alzheimer’s
researcher. “The plaques and tangles are the tombstones of the disease.”
The search for an exact cause has led scientists to suspect that a toxic
byproduct of a cell protein damages and kills nerve cells in the process
of forming plaques.
“Genetics points me in the direction of a molecule called a-beta
and a-beta biology,” Sisodia said. The UGA alumnus earned his doctorate
under the direction of Gordhan Patel, who is now UGA’s Vice President for Research.
Plaques form when a normal cell protein called APP — or amyloid
precursor protein — gets cut by enzymes in the wrong spot, releasing
a toxic fragment called a-beta peptide. The plaques themselves are not
thought to kill brain cells; scientists suspect that a wayward form of
the a-beta peptide is the culprit.
“The a-beta peptide not only accumulates in plaques but along the
way it will form these little fibrils that will be free-floating in the
brain,” said Rudolph E. Tanzi, professor of neurology at Harvard
Medical School and director of the genetics and aging research unit at
Massachusetts General Hospital. “The fibrils can interact with neurons
and cause problems, from blocking neural transmission to actually killing
nerve cells.”
Sisodia, Tanzi and many other researchers have hunted for genetic evidence
by turning to families where Alzheimer’s disease strikes early —
in the third or fourth decade of life, sometimes even earlier.
“The pathology in individuals who inherit these genes for early-onset
Alzheimer’s disease and the clinical symptoms are almost indistinguishable
— with some minor variations — from somebody who gets the disease
in the late onset form,” Sisodia said.
So far, the genetic clues support the notion that a-beta peptide production
in the brain is the probable cause of Alzheimer’s disease.
“The pathological cascade inside the cell revolves around the life
cycle of the a-beta peptide,” said Tanzi, co-author of Decoding
Darkness: The Search for the Genetic Causes of Alzheimer’s Disease.
The disease occurs when a person has defective genes that either make
too much APP or produce enzymes that cut APP in the wrong place, releasing
the “bad” a-beta. But a-beta production alone won’t cause
the plaques to form; a-beta has to clump together into fibrils to be toxic
to nerve cells. If enzymes chop up a-beta or if a-beta is shuttled into
the blood where it can be disposed of, clumping doesn’t occur, Tanzi
said.
“If too much a-beta is made at once and the machinery for clearing
it or degrading it can’t keep up, it’s going to form fibrils
more readily,” Tanzi said. “They’re going to cause neurotoxicity
and eventually it’s all going to manifest itself in senile plaques.”
Scientists still have much to learn about this bewildering disease that
kills the mind long before the body dies. Several UGA researchers are
contributing to understanding Alzheimer’s disease in a variety of
studies. They include the following:
• Cell biologist Marcus Fechheimer and his research
team developed a system to investigate abnormal cell structures called
Hirano bodies and what their role may be in Alzheimer’s and other
neurodegenerative diseases.
• Walter Schmidt, assistant professor of biochemistry and
molecular biology, studies several enzymes in yeast that bear similarities
to enzymes thought to have roles in Alzheimer’s and other diseases.
One of those enzymes is similar to insulysin — or IDE — which
may remove the a-beta peptide from the brain.
• Alan Przybyla’s biochemistry and molecular biology
lab has developed a method to produce a recombinant form of the a-beta
peptide. He helped form a start-up company, rPeptide, that provides researchers
around the world with molecules involved in Alzheimer’s, Parkinson’s
and other diseases.
• William Kisaalita, associate professor of biological engineering,
is developing cell-based tests, or biosensors, to screen drugs for Alzheimer’s
disease.
• Alvin Terry, associate professor of pharmacy, studies
the long-term effects of medication on the brain, particularly medications
commonly used to treat people with diseases that affect memory, like Alzheimer’s
disease and schizophrenia.
— KMC.
For comments or for information please e-mail the editor: jbp@ovpr.uga.edu
To contact the webmaster please email: ovprweb@uga.edu
![]()