

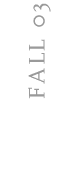
How
a Slime Mold
Came to the Aid of
Alzheimer's Research
by Kathleen Cason
Intro/Proteins
run amuck
| The path to hirano bodies
Serendipity and beyond
|
What's ahead

(A) Filaments made of the protein actin (red), as shown in this
mouse fibroblast cell, help cells move and eat, among other functions.
(B) Filaments made of tubulin (green) are like an intracellular
roadway in cells.
(C) Fechheimer’s lab forced cells like this mouse fibroblast
to make Hirano bodies (red spot) by inserting an altered form of the 34
kDa actin bundling protein into them. Hirano bodies are largely made of
actin, which is stained red in these cells.
Alzheimer’s Disease: 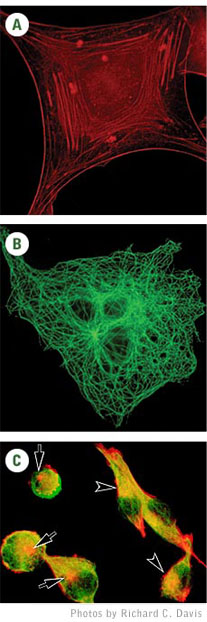
Inside the cell is a complex network of filaments made of distinct proteins
that give cells shape and provide a transportation system for moving materials
around.
A Checklist of Symptoms
Source:
www.alz.org
What's ahead
The scientists now had a way to investigate how Hirano bodies form, what they do and what their fate is: Insert the altered gene in a cell, and “presto” you’ve got Hirano bodies.
“Because it’s novel and a model system, you ask some very basic questions,” Furukawa said. “It’s hard to get to the more subtle questions until you figure out some basic biology.”
And basic questions abound: Do cells grow normally if they have Hirano bodies? What are Hirano bodies made of? What is their purpose?
So far the UGA team has discovered that slime mold cells with Hirano bodies grow normally or a bit more slowly but complete their life cycle.
“Further, mammalian cells with Hirano bodies also grow normally in culture,” Fechheimer said.
Hirano
bodies appear as almond-shaped protein deposits in brains of people with
Alzheimer’s and other neurodegenerative diseases (arrows point to
Hirano bodies).
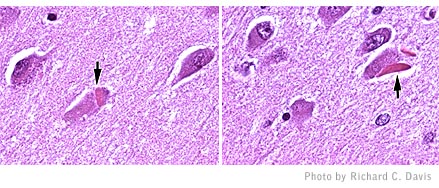
Unusual
protein deposits appeared in slime mold cells after an altered gene was
inserted into them. The deposits’ appearance and composition matched
the characteristics of Hirano bodies, an unusual structure that is found
in the brains of people with Alzheimer’s and other neurodegenerative
diseases. When observed with high magnification, a Hirano body (inserts
at right) appears like stacked wood from the “side” and “end”
views because of the arrangement of actin filaments. (Photo by Andrew
Maselli).
Hirano bodies are made of several hundred proteins; only a half dozen
or so have been identified. But which ones are required to make the deposits?
Graduate and undergraduate students are trying to find out, Furukawa said.
“Even though Hirano bodies look like they are mainly made of actin,
there are a lot of other things in there as well,” said doctoral
student Paul Griffin. “People speculate there might be growth factors
in there, transcription factors, all kinds of things. That’s what
I’d like to elucidate.”
But the big question is: What do these things do? Answering that question
may finally explain the link to Alzheimer’s and other brain disorders.
“The idea that Hirano bodies are simply a manifestation of cell
death is not being born out by our studies,” Fechheimer said.
Davis exposed cells that make Hirano bodies to oxidative stress to see
whether they live or die. Early evidence indicates that Hirano bodies
actually may have a protective function — bundling up damaged proteins
and spitting them out of the cell. Future studies by graduate student
Sang Deuk Ha will further probe the protective nature of Hirano bodies
in mammalian cells.
“We can make the Hirano bodies in Dicytostelium, in fibroblasts,
in epithelial cells, in nerve cells in culture,” Fechheimer said.
“So far the answer seems to be that Hirano bodies don’t hurt
these cells and perhaps they are protective.”
With one interesting twist.
When Davis introduced the altered bundling protein gene into HeLa cells
— cultured human cervical cancer cells — the cells died. In
fact, they underwent apoptosis, the scientific name for programmed cell
death. Intriguingly, HeLa cells do not appear to form Hirano bodies, unlike
the other cell types examined to date.
Fechheimer speculates that the modified HeLa cells undergo apoptosis
due to their failure to form Hirano bodies, further evidence to support
the idea that Hirano bodies may play a protective role. Now Davis, Ha
and undergraduate Dan Del Portal are investigating the relationship between
Hirano bodies and this type of cell death.
Still, it could be years before the link between Hirano bodies and neurodegenerative
diseases is better understood.
“It’s really about asking the question and solving the problem
in such a way that you get a clear answer,” Furukawa said. “When
you work in biology, you can ask every question you can think of but that
doesn’t mean you can think of a way to get an answer, and some straightforward
answer, not some murky answer.”
Future studies in the slime mold and other kinds of cells will uncover
what Hirano bodies are made of, how they form and whether they are essential
for certain cell processes. Knowing how Hirano bodies behave in cell culture
is not enough. For that reason, Fechheimer’s group also plans to
study them in entire organisms such as transgenic fish and mice.
“Our findings grew out of research funded by the National Science
Foundation,” said Fechheimer, who also received support from the
Alzheimer’s Association once the link to neurodegenerative disease
was established. “That’s why NSF is very excited by this connection
to something that sounded like brain disease. To them it was a wonderful
example why you never want to forget about investing in basic research.”
You never know what serendipitous discovery might be around the next
bend.
For more information, contact Marcus Fechheimer
at fechheim@cellmate.cb.uga.edu
or access www.uga.edu/cellbio/fechheimer.html
Kathleen Cason is associate director of Research Communications at
the University of Georgia.
Intro/Proteins
run amuck
| The path to hirano bodies
Serendipity and beyond | What's
ahead
For comments or for information please e-mail the editor: jbp@ovpr.uga.edu
To contact the webmaster please email: ovprweb@uga.edu
![]()